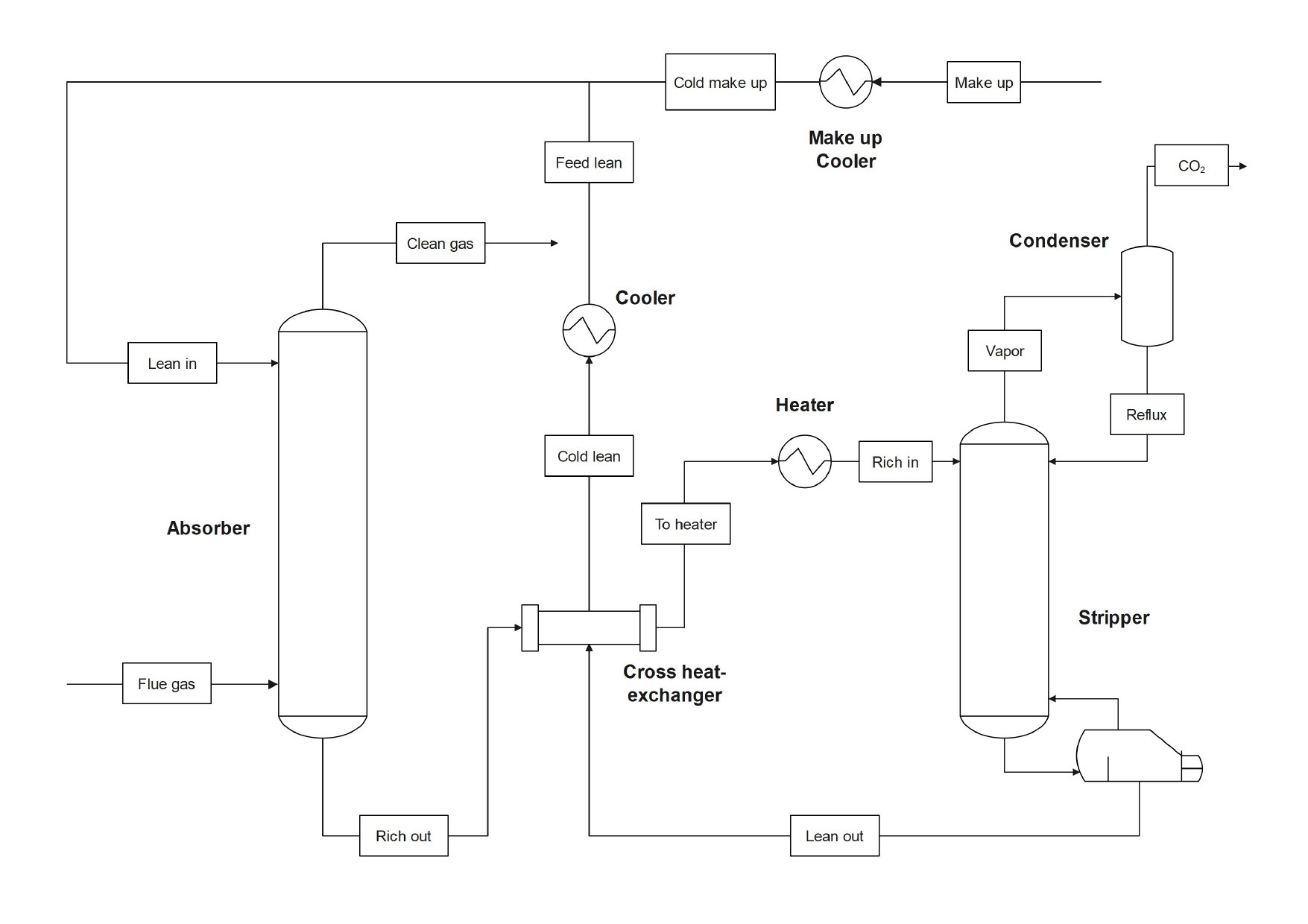
In this work, we address the production of urea from CO2 and ammonia because it allows for a high level of integration of energy and materials.
In this process, ammonia is produced via green hydrogen and nitrogen removed from the air, while CO2 can be sequestered from flue gases using aqueous ammonia as an absorbent. Thus, ammonia is used both as a reactant and sorbent.
We evaluate through simulation the direct use of the rich solvent in the urea synthesis loop, as compared with conventional urea production processes. Ammonia is obtained via a traditional Haber-Bosch reaction, hydrogen is created efficiently and at high purity from an electrolysis unit, and nitrogen is recovered from air via pressure-swing absorption.
A techno-economic analysis demonstrates that the total cost shows a substantial decrease compared to traditional processes, mainly because there is no need for solvent regeneration. In addition, the absorption efficiency of the new process is improved, because the lean solvent loading is zero. The fact that renewable energy sources can be used for nearly all units within the process means that the novel system provides a urea production solution which is both cheaper and has a smaller environmental footprint.
About us
Christopher Varela
Escuela Superior Politécnica del Litoral, ESPOL, Facultad de Ciencias Naturales y Matemáticas, Centro de Energías Renovables y Alternativas, Campus Gustavo Galindo, Km. 30.5 Vía Perimetral, Guayaquil, P.O Box 09-01-5863, Ecuador
Fernando Zea
Escuela Superior Politécnica del Litoral, ESPOL, Facultad de Ciencias Naturales y Matemáticas, Centro de Energías Renovables y Alternativas, Campus Gustavo Galindo, Km. 30.5 Vía Perimetral, Guayaquil, P.O Box 09-01-5863, Ecuador

Oris Correa
Department of Chemical and Petroleum Engineering, University of Kansas,1530 West 15th Street, Lawrence, KS 66045, USA

Kyle V. Camarda
Department of Chemical and Petroleum Engineering, University of Kansas,1530 West 15th Street, Lawrence, KS 66045, USA